The Magnetic Match
In this experiment, we’ll see how fire affects the magnetic properties of a matchstick’s head.
The process involves observing the matchstick before and after it’s burnt to understand the changes in its ability to interact with a magnet. This activity demonstrates the basic principles of chemistry and physics, offering a straightforward way to explore how the properties of materials can change through reactions, such as burning. It’s a simple investigation into the surprising behaviors of everyday materials under specific conditions.
Materials:
- Matches
- A strong magnet (Neodymium)
What to do?
- Try to lift the matchstick with the magnet. Are you successful?
- Now, light the matchstick and wait until most or all of it has burned.
- Try again to lift the matchstick with the magnet, were you successful this time?
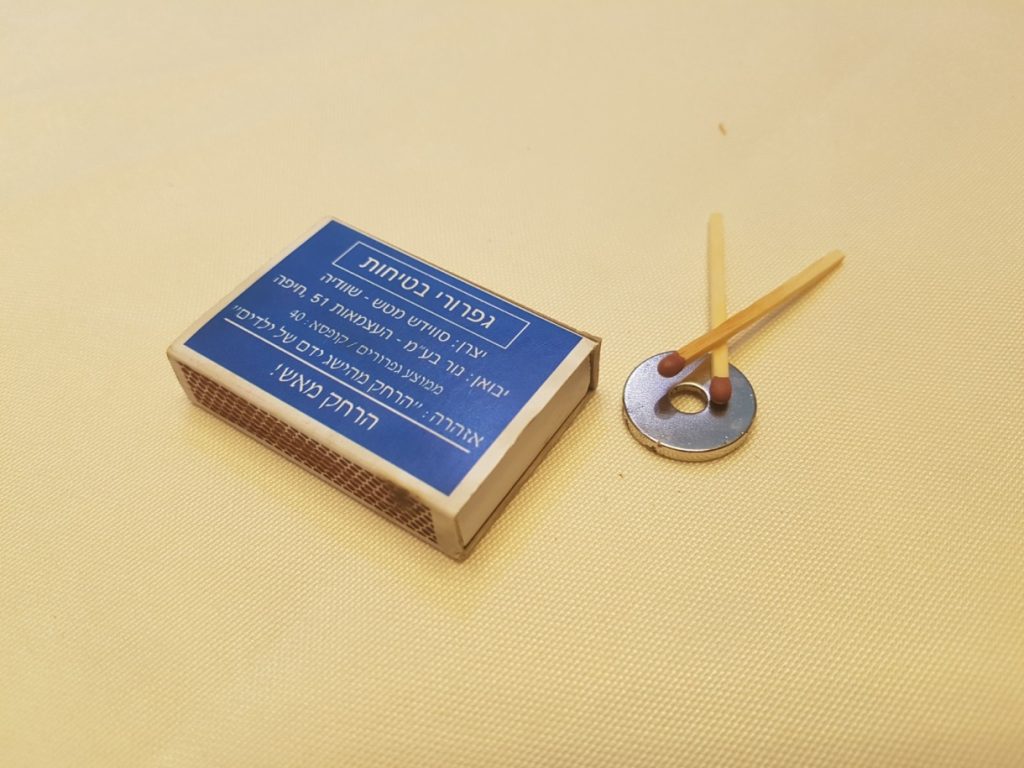

How does this happen?
The match head is made of a mixture of substances, the main one being potassium chlorate but also includes iron oxide (in a quantity of about 5-6%). If a magnet is brought close to a matchstick, it will not be attracted to it and will not become magnetized because iron oxide has very weak magnetism. However, during burning, a chemical reaction occurs in which the iron returns to its metallic state, and in this form, it has a stronger magnetic force. As mentioned, the amount of iron in a matchstick is small, so a very strong neodymium magnet must be used. Additionally, the more the matchstick burns, the lighter it will be, and the magnet will be able to lift it more easily.
We’d love to showcase your creativity!
Share pictures of your experiments with us, and together, we can inspire young scientists everywhere!